setTimeout(function(){
window.print();
},500)

Technical data Galvanic zinckingArticle no: P7402100 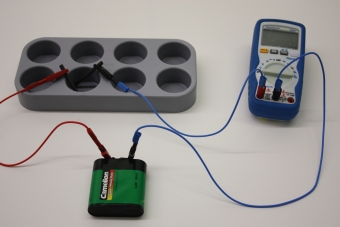 
<!DOCTYPE html PUBLIC "-//W3C//DTD XHTML 1.0 Transitional//EN"
"http://www.w3.org/TR/xhtml1/DTD/xhtml1-transitional.dtd">
Principle Technically, corrosion protection by zincking is attained by two different processes: By hot galvanizing, or by electroplating, i.e. by galvanic (electrochemical) deposition of zinc from a solution of zinc, in which the iron object to be treated is hung as the cathode. The electroplating procedure consumes considerably less zinc per square meter of surface than hot galvanizing, and is therefore now the preferred procedure.
Learning objectives
Benefits
Scope of delivery
| |||||||||||||||||||||||||||||||||||||||
PHYWE Systeme GmbH & Co. KG
Robert-Bosch-Breite 10 – 37079 Göttingen – Germany
www.phywe.com
Robert-Bosch-Breite 10 – 37079 Göttingen – Germany
www.phywe.com

