
Technical data A remarkable source of electric currentArticle no: P7400100 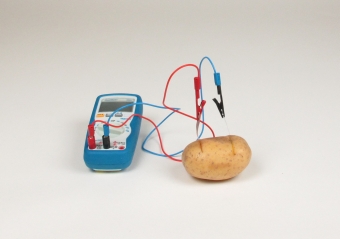
<!DOCTYPE html PUBLIC "-//W3C//DTD XHTML 1.0 Transitional//EN"
"http://www.w3.org/TR/xhtml1/DTD/xhtml1-transitional.dtd">
Prinicple The discovery and further development of galvanic elements, better known as batteries, is of great importance for humankind. It has enables mobile power supply of various electrical devices, which is a big part of our today's living standard. The functionality of a battery is based on a unifying principle - the difference in electrical standard potentials of metals and elements. The electrical current, produced by a battery, can be determined with the help of the standard potentials of the used metals. Students produce a galvanic cell by inserting a copper and zinc electrode into a potato. They measure the electrical current generated by the galvanic cell (potato battery) with a measuring instrument.
Learning objectives
Benefits
Scope of delivery
| ||||||||||||||||||||||||
Robert-Bosch-Breite 10 – 37079 Göttingen – Germany
www.phywe.com

