setTimeout(function(){
window.print();
},500)

Technical data Nonmetal galvanic cellsArticle no: P7400900 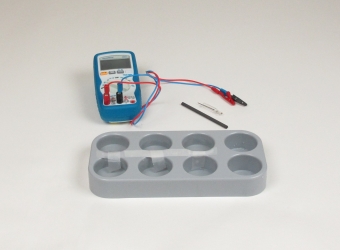
<!DOCTYPE html PUBLIC "-//W3C//DTD XHTML 1.0 Transitional//EN"
"http://www.w3.org/TR/xhtml1/DTD/xhtml1-transitional.dtd">
Principle In exactly the same way as metals, nonmetals also develop different solution pressures and so different potentials, as soon as they can form redox systems in appropriate solvents. In this experiment students prepare a simplified standard hydrogen electrode and also oxygen, chlorine, bromine and iodine half-cells. These half-cells are then to be combined to galvanic cells and their standard potentials measured.
Learning objectives
Benefits
Scope of delivery
| |||||||||||||||||||||||||||||||||
PHYWE Systeme GmbH & Co. KG
Robert-Bosch-Breite 10 – 37079 Göttingen – Germany
www.phywe.com
Robert-Bosch-Breite 10 – 37079 Göttingen – Germany
www.phywe.com